noble gas electron configuration|Noble Gas Configuration – Shorthand Electron Configuration : Baguio A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we . Confira agora as fotos e vídeos da gostosa Amiichan pelada em sua conta no OnlyFans. Essa musa do Erome aparece completamente nua, transando e fazendo sexo em vídeos vazados pelo Telegram e Reddit. Os nudes e packs dessa gata vão te deixar hipnotizado. Apenas maiores de 18 anos! Amiichan Porno em cenas de sexo
PH0 · Noble gas configuration
PH1 · Noble gas
PH2 · Noble Gas Shorthand
PH3 · Noble Gas Configuration – Shorthand Electron Configuration
PH4 · Khan Academy
PH5 · Electron Configuration With Noble Gas Notation
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Electron Configuration
PH8 · 5.20: Noble Gas Configuration
PH9 · 2.8: Electron Configurations
Midas Furniture was founded in 1993 on the belief that customers seeking premium furnishing solutions in the Middle East region deserve more. Our vision continues to be focused on bringing a unique and revolutionary concept of furniture retail that provides our customers with a special furniture shopping experience characterized by elegance, .
noble gas electron configuration*******Learn how to write a noble gas configuration for any atom using the Aufbau principle and the periodic table. See examples and a list of noble gas configurations for all 118 elements. Tingnan ang higit pa
Noble Gas Configuration – Shorthand Electron ConfigurationThe noble gas configuration gives the noble gas core that occurs before the element on the periodic table and then the electron configuration of the atom’s valence electrons. But, you need to understand how to write the full electron configuration . Tingnan ang higit paFor example, write the noble gas configuration of sodium. 1. The atomic number of sodium is 11, so you know the neutral atom has 11 protons and also 11 electrons. 2. Filling in the electron shells using the Aufbau principle gives a configuration . Tingnan ang higit paA noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we . Mar 23, 2023 In this video, you will learn how to write the electron configurations of atoms using the noble gas notation. You will also see some examples of how to apply this notation to different elements .
Learn how to write electron configurations for atoms and ions using noble gas configuration. Watch a video lesson and practice exercises on Khan Academy's free online . Learn how to write the electron configuration of an element using noble gas notation with this chemistry video by The Organic Chemistry Tutor. See examples, rules, and .Learn how to write the noble gas shorthand for any element using the periodic table. See examples, definitions, and practice problems for noble gas electron configurations.
Noble gases are the elements of group 18 with full outer shells of valence electrons. They are odorless, colorless, monatomic gases with very low reactivity and cryogenic boiling points. Learn about their history, discovery, and uses.
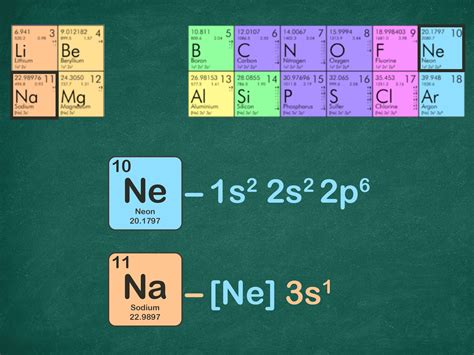
Noble Gas Configuration. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner .
noble gas electron configuration The noble gas core configuration is an abbreviated notation of an atom's electron configuration. Here, the noble gas's electron configuration is substituted and replaced with the noble.

Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.Noble gases have eight electrons in their outermost shell, except in the case of helium, which has two. The noble gases are colorless, . As a result of a full shell, the noble gases can be used in conjunction with the electron . Noble Gas Configuration. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\). The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. . (Kr) is the previous noble gas listed before iodine. The noble gas configuration encompases the energy states lower .noble gas electron configuration Noble Gas Configuration – Shorthand Electron Configuration Courses on Khan Academy are always 100% free. Start practicing—and saving your progress—now: https://www.khanacademy.org/science/chemistry/electronic-struct. Put the brackets around the electron configuration of the last noble gas, group 18 element, before nitrogen. Note that helium (He) is the noble gas preceding nitrogen. Continue with the electron configuration after helium. So for nitrogen, it would look like this: [H e] 2 s 2 2 p 3 [{\rm He}]\rm 2s^22p^3 [He] 2 s 2 2 p 3.A Noble Gas is a group of elements that in their standard state have a filled electron cloud.. These elements are found in the 18th column of the periodic table and include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn). They are all odourless and colourless mono-atomic elements. Because these elements are already electron stable and do not need .Why is it possible to abbreviate electron configurations with a noble gas in the noble gas notation? 4. Identify the following elements: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6; . A noble gases with f electrons; (c) a fifth-period element whose atoms have three unpaired p electrons; (d) First rowtransition metals having one 4s electron. Answers. 1 .All of the electrons in the noble gas neon (atomic number 10) are paired, . The valence electrons (those added after the last noble gas configuration) in these elements include the ns and (n – 1) d electrons. The official IUPAC definition of transition elements specifies those with partially filled d orbitals.The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1s 2 2s 2 2p 6 3s 2 3p 6, the same as that of the noble-gas argon. The sodium ions are sodium atoms which have lost an electron, giving them the structure 1s 2 2s 2 2p 6, the same as that of the noble-gas neon. All electrons in both kinds . The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the .
This row concludes with the noble gas argon, which has the electron configuration [Ne]3s 2 3p 6, corresponding to a filled valence shell. Example \(\PageIndex{1}\): Electronic Configuration of Phosphorus . Ignore the inner orbitals (those that correspond to the electron configuration of the nearest noble gas) and write the valence electron .
The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in a condensed . The noble gas core configuration is an abbreviated notation of an atom's electron configuration. Here, the noble gas's electron configuration is substituted and replaced with the noble gas's . The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the .Potassium has nineteen electrons, one more than the noble gas argon, so its configuration could be written as \(\left[ \ce{Ar} \right] 4s^1\). In a similar fashion, strontium has two more electrons than the noble gas krypton, which would allow us to write its electron configuration as \(\left[ \ce{Kr} \right] 5s^2\). Noble Gas Configuration. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{1}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\).
This chemistry video explains how to write the electron configuration of an element using noble gas notation.Speed of Light, Frequency, Wavelength: http.
Keep your CV under two pages: As a general rule of thumb, the length of your CV should not exceed two A4 pages. Employers prefer short CVs because they are easier to scan/read and contain only the most .
noble gas electron configuration|Noble Gas Configuration – Shorthand Electron Configuration